The tube is bent into a "U" shape and mounted on an inexpensive piezoelectric speaker. The resonance frequency of the tube is detected by a photointerrupter, amplified, and fed into the speaker; the resulting feedback circuit keeps the glass tube vibrating at its resonance frequency. A peristaltic pump and computer are used to flow samples through the tube and record the resonance frequency of the tube. Detail of the glass tube shows the path followed by a sample inside the tube as it flows into the sensor ("a"), passes the tip of the sensor ("b"), and exits the sensor ("c").
Resonance frequency of the vibrating tube vs. time as a 770 μm diameter glass bead is passed back and forth through the tube eighteen times. Each passage of the bead through the tube results in a momentary decrease in the tube's resonance frequency; this is recorded as a downward peak in the resonance frequency . Each point on the peak (baseline "a," tip "b," and baseline "c") corresponds with the bead's location in above. The height of this peak is used to determine the buoyant mass of the bead .
Histograms showing the buoyant mass of another bead weighed thousands of times in two different fluid densities. In deionized water (density 1.000 g/mL) the bead has an average buoyant mass of 3.69 ±0.12 μg, and in a sodium chloride solution (density 1.046 g/mL) the bead has an average buoyant mass of −4.42 ±0.16 μg. The widths of these distributions—120 and 160 nanograms—provide an estimate of the resolution of our mass measurements. In this work, we demonstrate a simple and inexpensive sensor capable of weighing microgram-sized objects in fluid. Like the SMR, this sensor uses a change in resonance frequency to weigh an object in fluid with high precision. But unlike the SMR, this sensor can weigh samples with a large range of sizes and is extremely simple to fabricate.
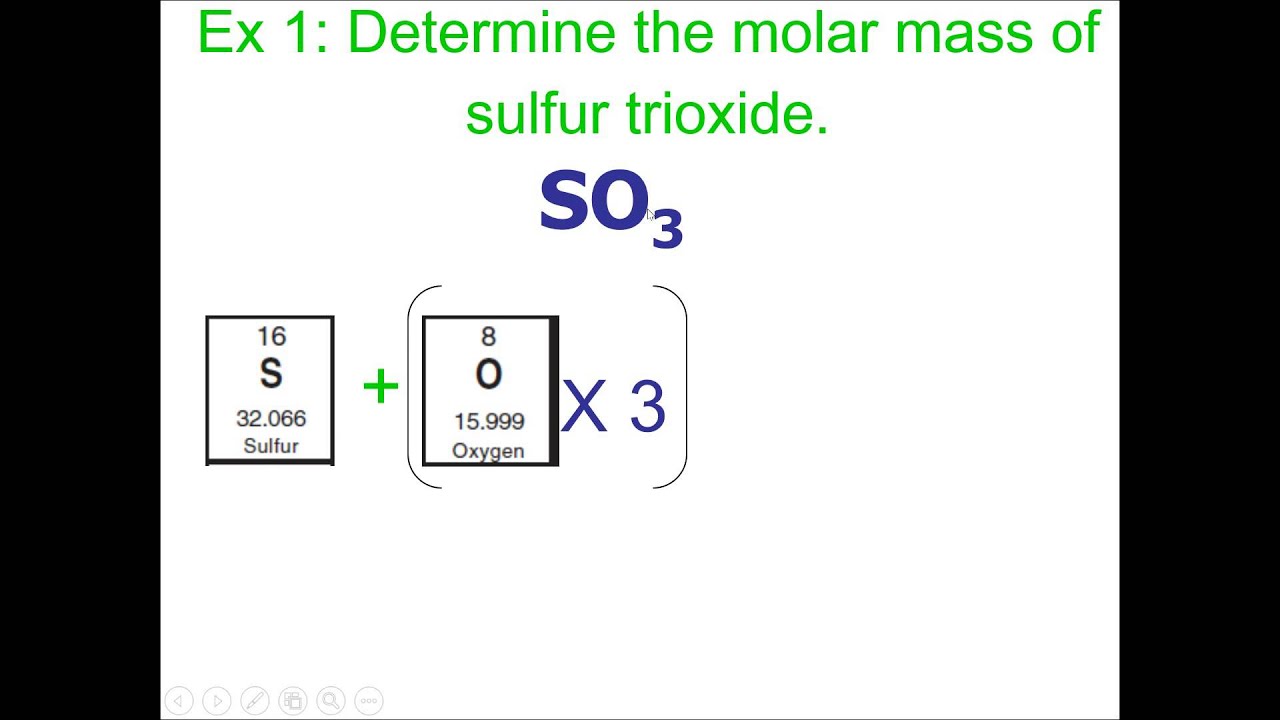
Our sensor consists of a short length of glass tubing bent into a "U" shape and attached to an inexpensive speaker that vibrates the glass tubing at its resonance frequency. The resulting sensor shown in Fig 1 costs about US $12 in materials and can be made in under 10 minutes. Additionally, by weighing samples in fluids of different densities, we can also use our sensor to measure the volume and density of samples in fluid. The four proof-of-concept samples studied here—microbeads, embryos, seeds, and biomaterials—are representative of a wide range of samples that may be analyzed in fluid using vibrating glass tube sensors. Our technique is very versatile because all objects have fundamental physical properties like mass.
Consequently, our mass sensor can be applied to problems as diverse as screening toxic substances, understanding the growth of plants, measuring the degradation of biomaterials, and many others. And unlike imaging-based measurements of size, our mass sensor is insensitive to the shape of the object. Finally, the automation, portability, and low cost of this technique make vibrating glass tubes particularly well suited for applications in the field or in resource-limited settings. When a sample passes through the vibrating tube sensor, its buoyant mass is recorded as a brief peak in the plot of resonance frequency vs. time (e.g., Fig 1C and 1D).
Once a peak is located, the height of the peak can be measured and converted to a corresponding buoyant mass value using the sensor's point mass calibration described below. Alternatively, a custom Python program can be used to fit the raw frequency measurements to an analytical equation of expected peak shape derived from Dohn et al. The resulting buoyant mass measurements were recorded and processed using a moving window average filter with a window size of five data points to slightly reduce noise in the plots of buoyant mass vs. time. Additional details about signal processing are provided in S6 Fig. Before using a vibrating tube to weigh a sample, the tube must first be calibrated.
We calibrated the sensor shown in Fig 1A using a glass bead of known size and density in a fluid of known density . Fig 1C shows eighteen downward peaks in the resonance frequency of the tube as the bead is pumped back and forth eighteen times through the tube. The height of each peak (72 millihertz; Fig 1D) is proportional to the buoyant mass of the bead . Our homemade vibrating tube mass sensors typically have quality factors around 500, which is comparable to that of tuning forks and high enough for precise measurement of the tube's resonance frequency . Where mo is the absolute mass of the object, ρo is the density of the object, and ρf is the density of the fluid filling the channel. Stated in words, an object's buoyant mass is equal to its real mass minus the mass of an equivalent-volume amount of fluid.
If the object's density is less than the fluid's density, then the object has a negative buoyant mass and its passage through the tube will result in a momentary increase in the tube's resonance frequency . Finally, if the object's density equals the fluid's density, then the object will have zero buoyant mass and its passage through the tube will have no effect on the resonance frequency of the tube . Note that while the vibrating tube sensor is sensitive to an object's buoyant mass, it is not affected by buoyant forces because the object being measured is confined to the tube and cannot sink or float vertically.
Thus, the orientation of the vibrating tube with respect to gravity has no effect on its measurements. Measurements of an object's fundamental physical properties like mass, volume, and density can offer valuable insights into the composition and state of the object. However, many important biological samples reside in a liquid environment where it is difficult to accurately measure their physical properties. Ρ is the object's density m is the object's total mass V is the object's total volume Under specified conditions of temperature and pressure, the density of a fluid is defined as described above.
How To Find Density With Volume And Weight However, the density of a solid material can be defined in several ways. Porous or granular materials have a density of the solid material, as well as a bulk density, which can be variable. For example, if you gently fill a container with sand, and divide the mass of sand by the container volume you get a value termed loose bulk density. If you took this same container and tapped on it repeatedly, allowing the sand to settle and pack together, and then calculate the results, you get a value termed tapped or packed bulk density. Tapped bulk density is always greater than or equal to loose bulk density. In both types of bulk density, some of the volume is taken up by the spaces between the grains of sand.
The density of the sand grains, exclusive of the air between the grains, will be higher than the bulk density. If the object is regular, you can use a volume formula. For example, the volume of a rectangular prism equals width x height x length. To make this calculation, measure the width, height and length of the object in centimeters. You can also use a graduated cylinder to measure volume.
Simply fill the graduated cylinder with water and note this measurement. Find the difference between the new and original measurements on the graduated cylinder. For your density calculation, you will need a volume in cubic centimeters, so convert units accordingly. Raw resonance frequency data from repeated measurements of a single polyethylene microbead in water (from the right-hand distribution of measurements in Fig 1E). After zooming in to the filtered data , peaks corresponding to individual measurements of the microbead are visible.
Zooming in further on one pair of peaks shows the ∼2 millihertz height of these peaks (corresponding to a buoyant mass of ∼3.7 μg for this microbead). The peaks come in pairs because the particular vibrating tube sensor used for this measurement had a tuning-fork shape with two vibrating "U"-shaped lobes . As an object passes through the vibrating tube sensor, the shape of each resulting peak in the tube's resonance frequency is a function of the vibrational mode and amplitude of the tube. In this work, the tubes are vibrating at their primary vibrational mode, meaning that the amplitude of vibration is highest at the tip (the bottom of the glass "U") and lowest at the base (the top of the "U"). As the particle leaves the tube (point "c" in Fig 1B) the tube's vibrational amplitude in this region decreases again, so the resonance frequency of the tube returns to baseline (point "c" in Fig 1D). General mathematical expressions for predicting this peak shape for any vibrational mode were derived by Dohn et al.
Magnesium ribbon with a thickness of 250 μm (98% pure; MiniScience Inc., Clifton, NJ) was used as a model biomaterial in our degradation rate measurement studies. Roughly 1 mm sized pieces of magnesium were cut from the ribbon. The samples were polished before measurement using 600, 800, and 1200 grit silicon carbide abrasive papers to remove the native oxide layer. Flow through the sensor was controlled using the servomotor as described previously. The resulting buoyant mass measurements are shown in Fig 4.
What two things do you need to know in order to find the density of water? Students should realize that they need both the volume and mass of a sample of water to find its density. Suggest that students use a graduated cylinder to measure volume in milliliters. Suggest that students use a balance to measure the mass in grams. Tell students that they can find mass by weighing the water. However, since water is a liquid, it needs to be in some sort of container.
So in order to weigh the water, they have to weigh the container, too. Explain to students that they will have to subtract the mass of an empty graduated cylinder from the mass of the cylinder and water to get the mass of just the water. The density of a substance can be used to define the substance.Water is unusual because when water freezes, its solid form is less dense than liquid water, and thus floats on top of liquid water. In Fig 1C a slight downward drift is visible in the baseline resonance frequency of the vibrating tube over time; the frequency is decreasing at about 2 millihertz per minute. Slow baseline frequency drift like this is common in vibrating mass sensors and is mostly due to small fluctuations in the temperature of the sensor. However, the magnitude of this drift is small compared to the duration of a peak, which is why the baseline of an individual peak in Fig 1D is relatively flat.
Furthermore, the terms use different units to represent their respective conditions. In the case of density, this is a compound unit – both the unit of mass and the unit of volume are mentioned. For example, grams per cubic centimeter (g/cm3) or pounds per cubic foot (lb/ft3) are the often-used units for measurement of density. On the other hand, due to gravity's influence on weight – it, weight is expressed by Newton's law (symbolized by a capital "N"),. The most accurate way to calculate the density of any solid, liquid or gas is to divide its mass in kilograms by its volume (length × width × height) in cubic metres.
The density of water is approximately 1000 kg/m 3 and the density of air is approximately 1.2 kg/m 3. Of a substance is the ratio of the mass of a sample of the substance to its volume. The SI unit for density is the kilogram per cubic meter (kg/m3). For many situations, however, this as an inconvenient unit, and we often use grams per cubic centimeter (g/cm3) for the densities of solids and liquids, and grams per liter (g/L) for gases. Although there are exceptions, most liquids and solids have densities that range from about 0.7 g/cm3 to 19 g/cm3 .
Table \(\PageIndex\) shows the densities of some common substances. The density of any material refers to how compact a certain object is. If placed in a mathematical equation, you determine this property by the mass contained within a given volume. We've heard about measurements like grams per cubic centimetre or pounds per square foot. Additional measurements of the buoyant mass of single zebrafish embryos in water, obtained using our vibrating glass tube sensor.
Distribution of buoyant mass measurements for 474 different zebrafish embryos at 2 hours post-fertilization, obtained using our vibrating glass tube sensor. To validate our technique with a diverse range of biological samples, we used vibrating tube sensors to monitor the mass of individual plant seeds during imbibition and germination. Seed imbibition, or water uptake, is used in agriculture as a metric of seed health and quality .
Seed germination is a change in seed metabolism when imbibition starts; germination culminates with the elongation of the embryonic axis that penetrates the seed coating. The imbibition of seeds is accompanied by a rapid leakage of cellular materials and the rate of this leakage is decreased as the tissues become hydrated . If water uptake by the seed is too rapid, the seed tissue might experience injury, and if the seed enters an anaerobic state, the seed might experience accumulation of toxic chemicals such as ethanol. Both situations can encourage undesirable seed dormancy and delay germination .
In summary, seed imbibition and germination are important phenomena in plant research, and quantitative measurements of these phenomena would be valuable in a wide range of botanical and agricultural fields. Additional measurements of the buoyant mass of single zebrafish embryos exposed to various known toxicants, obtained using our vibrating glass tube sensor. Finally, to demonstrate our technique using a biologically-relevant sample other than an organism, we used vibrating glass tubes to precisely measure the degradation rates of biodegradable materials. For many applications in medical implants, it is desirable to have materials with known degradation rates. For example, a screw for repairing a broken bone might remain intact until the bone heals and then dissolve away. However, measuring the degradation rates of slow-degrading materials is a time-consuming and labor-intensive process.
This process slows the development of new biomaterials and introduces the potential for human error. To determine the resolution of our technique, we used another glass tube to make 4,483 measurements of the buoyant mass of another bead of known size and density in water. The width of the distribution of these measurements (120 nanograms in the right-hand distribution in Fig 1E) represents the smallest difference or change in mass that can be detected by this glass tube. An object's density is defined as the ratio of mass to volume. Density is used across geology, physics, and many other physical sciences. The property also determines whether or not an object would float in water, which has a unit density of 1 gram per cubic centimeter (g/cm3)—the standard units for density measurements.
Because the density of water in g/cm3 is 1.0, the SG of an object is will be almost the same as its density in g/cm3. However, specific gravity is a unitless number, and is the same in the metric system or any other measurement system. It is very useful when comparing the density of two objects.
Since specific gravity is unitless, it doesn't matter whether the density was measured in g/cm3 or in some other units (like lbs/ft3). If the value is in cubic inches, divide the number by 1,728 cubic inches, then divide the weight by this number. You may now divide your measurements accordingly to find this density.
Depending upon the units of the household scale/container you used, it may be necessary to do some conversion to get your object's density in the standard, meaningful units of kilograms per cubic meter, kg/m3. By using our resonating glass tube to automatically monitor the mass of a degrading material in fluid, we can use a much smaller sample of material than would normally be required. These small (millimeter-sized) samples have a much larger surface-area-to-volume ratio than the centimeter-sized samples required by current methods. This increases the relative degradation rate of the smaller samples in fluid, making our technique capable of measuring the degradation rate of a material in hours instead of weeks or months. We used our sensors to measure the degradation rate of a sample of magnesium, a biodegradable material that has been extensively studied for potential use in medical implants .
To test this hypothesis, we used vibrating glass tubes to continuously monitor the buoyant mass of zebrafish embryos during exposure to known toxicants. Zebrafish embryos are popular vertebrate model organisms for high throughput drug discovery and screening and human disease modeling . Additionally, as marine animals, they are used extensively in assessing the toxicity of substances in aquatic environments .
Current methods for assessing the health of zebrafish embryo are laborious, time-consuming, require a high degree of expertise, and can be subject to human error. Vibrating tube mass sensors could offer an economical and high-throughput alternative to these existing techniques for assessing the health of an embryo. They can also provide information on organism mass, a primary metric in toxicology that is used as a normalizing factor for dosing of toxicants . Seeds of iceland poppy , oregano , and foxglove were obtained from Ferry-Morse and measured every 10 seconds in water until active germination was observed using the servomotor reservoir lifter described above. This estimate of single-seed mass was used along with the seed buoyant mass measurements to calculate the estimated density of each seed during imbibition and germination (Fig 3G–3I).
However, there are many important samples that are too large to be weighed in the microfluidic SMR and too small to be weighed on a conventional balance. For example, fish embryos are used in a variety of developmental biology and toxicology applications . If the weight of an embryo could be monitored as it grows or reacts to stimuli, these measurements could offer new insights into developmental biology and toxicology. However, millimeter-sized zebrafish embryos are far too large for the micron-scale channels in the SMR, and the aquatic environment of the embryos complicates weighing them using a traditional balance. But accurately predicting the degradation rates of these materials in the fluidic environment of the body is challenging. Argon - Density and Specific Weight - Online calculator, figures and tables showing density and specific weight of argon, Ar, at varying temperature and pressure - Imperial and SI Units.
The soil bulk density , also known as dry bulk density, is the weight of dry soil divided by the total soil volume . The total soil volume is the combined volume of solids and pores which may contain air or water , or both . The average values of air, water and solid in soil are easily measured and are a useful indication of a soils physical condition.






















No comments:
Post a Comment
Note: Only a member of this blog may post a comment.